By Chris Black
Who is Prof. Dr. Christian Drosten?
Prof. Drosten develops the SARS-CoV-2 test and immediately publishes its instructions through the WHO, in breach of his professional obligation to notify Charité Hospital (his employer) of inventions, so that Charité cannot apply for a possible patent.
At the same time, however, Prof. Drosten collaborated from the very beginning with a Berlin based company, TIB Molbiol Syntheselabor, which sends the test to laboratories around the world with which Prof. Drosten is in contact. TIB Molbiol has a massive competitive advantage here, being the first manufacturer of the test.
Amazing thing: Prof. Drosten and TIB Molbiol have always collaborated in exactly the same way with all recent “viruses” (SARS, MERS, bird flu, swine flu): Prof. Drosten develops the test and shouts in public about the danger of the virus and the dire need for mass testing , and then TIB Molbiol, which works with him directly, is the first in the world to produce mass test kits and thus earns a lot of money.
What happens here?
The Curriculum Vitae of Mr. Drosten from 2017 that was published on the website of the German Medical College looks like this and claims that he would have given his doctorate in 2003.
In another CV, written by him, published on the page European Network for Diagnostics of “Imported” Viral Diseases (ENIVD), the date of the doctorate appears to be in the year 2000.
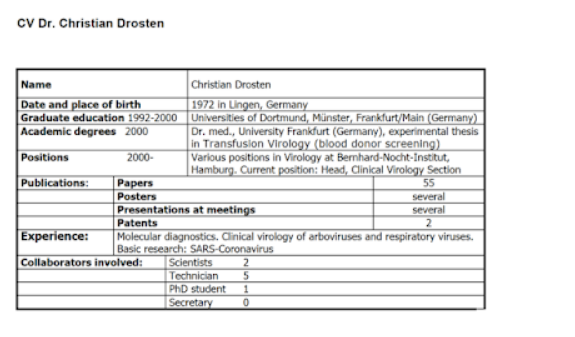
The theme of the paper according to this CV is “experimental thesis in Transfusion Virology (blood donor screening)”.
On Charité’s Drosten Research Laboratory page, he names his work “Aufbau der ersten Testsysteme für HIV ‑ 1 und HBV im Hochdurchsatzverfahren” (“Establishment of the first high-capacity HIV-1 and HBV testing systems”). There he also spreads his claim that he was the only discoverer of SARS in 2003.
Where is Drosten’s doctoral dissertation?
At the university library in Frankfurt in June 2020 there was only a summary and index of the work, which strangely seemed to be accessible only in the reading room of the library. The two copies available in the library were “borrowed” (not available) and there was no waiting list for those who were interested in reading them.
Also, in the catalogue of the German National Library for the years 2000-2003 there is no mention of any doctoral thesis by Mr. Christian Drosten.
If you search on the National Library of Medicine page (pubmed, you know, the favourite site of scientists), with the keywords “Drosten + HIV”, you will find 16 papers, but here too the doctoral thesis is missing in action. The paper does not appear among the works on HBV either.
But here is the miracle: a month later, in JULY 2020, Drosten’s work suddenly appears in the catalogue of the German National Library. But guess what: according to an accompanying technical document (BIBFRAME blablabla), the work (created in a few weeks) was uploaded to the site only in July 2020:
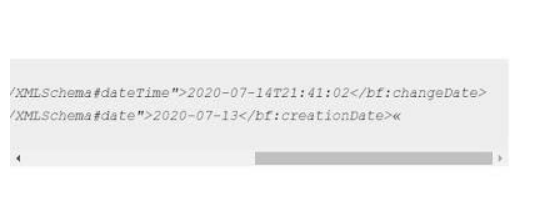
A German researcher specializing in scientific fraud, Dr. Markus Kühbacher, found that Mr Drosten’s so-called doctoral dissertation was NOT delivered in 2003, the date on which it was officially claimed that it had been handed over, and the University of Frankfurt admitted she lied, because she was missing the famous “Revisionsschein” (revision certificate), which is mandatory when submitting doctoral dissertations.
As “Professor” Drosten is the subject of several criminal proceedings (Lanka, Füllmich & Co., Kühbacher), we look forward to the outcome.
Mr. Drosten and the PCR testing business
We return to the fantastic duo: the Christian Drosten pharma darling and the TIB Molbiol Syntheselabor company, which have been a very hard-working team since 2003. TIB Molbiol offered Charité Hospital (Drosten’s employer) “logistical support” to ship samples of SARS-CoV-2 test kits to laboratories in Thailand, Vietnam, Hong Kong, etc. Obviously, the (paid) subsequent orders were taken over directly by TIB Molbiol.
In fact, the diligent “professor” Drosten is always the first to fight “viruses”, as follows: SARS-CoV (2003), bird flu (2005), swine flu (2009), Chikungunya virus (2009), MERS (2012), Zika (2016), yellow fever in Brazil (2017), and lastly SARS-CoV-2 (2020).
In terms of legal issues, such as patents, the test developed in 2003 with Mr Drosten’s contribution to SARS-CoV was urgently patented by the company Artus GmbH (which collaborated with Drosten’s then employer, the Bernhard Nocht Institute). But in 2020, Mr Drosten’s current employer, the Charité Institute in Berlin, says:
“Professor Drosten was commissioned by Charité to develop the PCR test, which he worked on, and which was funded by the Ministry of Education and Research, as well as by the European Union. These funds were specifically earmarked for the development of tests for new viruses. “
We must ask: did the Charité Institute secure its commercial rights by applying for a patent before submitting the test protocol to the WHO? When asked about Mr Drosten’s collaboration with the TIB Molbiol company, the Charité Institute replied:
“There were no agreements; neither side intends to demand rights. The collaboration took place strictly for humanitarian reasons.”
However, it seems that, ironically, the Charité Institute was left hanging out to dry, because the director of TIB Molbiol, Olfert Landt, had no problem with collecting huge sums of money from the sale of millions of kits of SARS-CoV-2 tests, for which Landt’s own son was co-opted into the company.
This is big business for a company that already had an annual profit of 7.3 million euros, and in 2020 the profit will be 9 times higher according to Landt’s calculations.
“Professor” Drosten has been collaborating with Olfert Landt for 17 years, and they have even published 11 “scientific” papers together. However, it is a bit sad to read that Olfert Landt states that there is no friendship between them, and he was “absolutely accidentally” at the Institut Charité in January 2020, just as Drosten was working on the development of the PCR test for SARS-CoV-2.
As such, since the “plandemic”, the TIP Molbiol company is getting rich by millions upon millions, and Drosten and the Charité Institute were left holding the bag.
The managers of the Charité Institute were asked:
“With regard to the so-called Drosten test, its components, the primer (ie the start-up sequence), etc., did the Charité Institute submit any application or participated directly or indirectly in such an application?”
Their response was as follows (very relevant!!):
“No. Professor Drosten did not develop a test kit, but published – in the form of a procedural protocol – the information crucial for laboratories to perform a test. Companies have developed test kits on this basis. Professor Drosten does not receive any income from this.”
So Drosten did not develop any tests, but only a testing protocol for laboratories around the world? This sounds a bit elusive (in the direction of fraud): if an employee of Volkswagen uploads a manufacturing protocol for a dust filter and then states that he did not invent or develop anything, but only created a protocol for manufacturing, possibly for humanitarian reasons, to help humanity get rid of the dust, I think the legal department of Volkswagen would not be happy.
In the case of the SARS-CoV-2 test, the protocol already contains absolutely everything a laboratory needs to produce its own test kits and sell them to others.
By March 2020, Olfert Landt’s TIB Molbiol had already produced and sold three million tests in more than 60 countries. The first ones had already been sent to Hong Kong in January, without instruction manuals, which were later sent by e-mail, even though the Charité Institute definitely had documentation for that.
Since 2003, legacy media has been introducing us to the charming couple: the modest genius virologist Christian Drosten together with the modest entrepreneur Olfert Landt, the unsung heroes of the modern world.
With each plandemic, like the SARS-CoV (2003), bird flu (2005), swine flu (2009), Chikungunya virus (2009), MERS (2012), Zika (2016), yellow fever in Brazil (2017) , and finally SARS-CoV-2 (2020), TIB Molbiol made out like bandits.
We must remember the swine flu hysteria, when tens of thousands of potential deaths had been prophesised by mass media, yet in the end in Germany, the deaths attributed as being due to swine flu remained below 500.
Here’s how Drosten’s business friend Olfert Landt openly recounts in a March 2020 interview with the Berliner Zeitung how test fraud works:
“The test design, its development comes from the Institute Charité. We just put everything in a test kit format. And when you don’t have the virus, which at first was only in Wuhan, we can produce a synthetic gene to simulate the virus genome, which we did very quickly. The validation study was published on 23 January in the journal Eurosurveillance by Corman et al. “
Well, what do you do when that validation study is actually written by Olfert Landt with Christian Drosten, who also appears as a contact person? What do you do when Drosten and Landt had already published a “preliminary protocol and assessment” for the WHO 10 days before, in which Drosten was also a contact person?
Further in the interview with the Berliner Zeitung:
“Mr Landt, we must still mention that you were involved in the research process, and now you do a lot of business with tests, although science should be independent. You do not have a conflict of interest? “
Landt responds:
“It’s perfectly reasonable to put certain things in the hands of companies – they have a financial incentive to provide a service, which is what drug manufacturers do. The drug is good because it heals people, the pharmaceutical company makes money with it. This is a completely normal situation and I don’t find it at all reprehensible. “
As for earning money, during his first interview with Taz magazine, Landt stated that a PCR test for covid sells for 2.50 euros. In other words, if laboratories in Europe charge up to 300 euros per test, that’s not his fault anymore. He can produce 500,000 tests daily, which leads to a nice profit.
On the same day as the interview for Taz magazine, Landt spoke on Deutschlandfunk radio and there the test already cost 10 euros 😉 And the Federal Insurance Companies Authority is already talking about a sum of 59 euros for the settlement of a “nucleic acid detection test to the beta-Coronavirus SARS-CoV-2 “.
Meanwhile, on 27 November 2020, 22 scientists asked the Eurosurveillance journal to WITHDRAW the article from 23 January 2020, co-authored by Drosten- Charité and Landt / Kaiser – TIB Molbiol:
“Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR“, which constitutes from their point of view the entire unscientific basis of PCR tests.
I give you below from the evaluation of the Corman-Drosten paper the FATAL problems of this paper, highlighted by the 22 scientists:
“The external peer review evaluation of the RTPCR test for the detection of SARS-CoV-2 shows 10 major scientific errors at the molecular and methodological level: the consequences for false-positive results.”
- There is no specific reason for using extremely high concentrations of primers in this Protocol. The concentrations described lead to a large number of non-specific binding and amplification of the product by PCR, making the test completely unsuitable as a specific diagnostic tool for the identification of SARS-CoV-2 virus.
- Six unspecified, unstable positions will introduce enormous variability in real-world laboratory implementations of this test; the totally confusing non-specific description in Corman-Drosten’s work is not adequate as a standard operational protocol, making the test inadequate as a specific diagnostic tool for the identification of SARS-CoV-2 virus.
- The test cannot distinguish between the whole virus and the viral fragments. Therefore, the test cannot be used as a diagnosis for intact viruses, as it is inadequate as a specific diagnostic tool to identify the SARS-CoV-2 virus and to assume the presence of an infection.
- A difference of 10 ° C in the malleability temperature Tm for the primer pair1 (RdRp_SARSr_F and RdRp_SARSr_R) also makes the test inadequate as a specific diagnostic tool for the identification of SARS-CoV-2 virus.
- A severe error is the omission of an exact Ct value, at which a sample is considered positive and negative, respectively. This Ct value is not found in subsequent additions, which makes the test not suitable as a specific diagnostic tool for the identification of SARS-CoV-2 virus.
- PCR products have not been validated at the molecular level. This makes the protocol useless as a specific diagnostic tool for identifying the SARS-CoV-2 virus.
- The PCR test contains no single positive control to assess its specificity for SARS-CoV-2, nor a negative control to rule out the presence of other coronaviruses, making the test completely inappropriate as a specific diagnostic tool for the identification of SARS-CoV-2. CoV-2 virus.
- The design of the test in Corman-Drosten’s work is so vague and flawed that it can go in dozens of different directions; nothing is standardized and there is no Standard Operating Procedure (POS). This calls into question the scientific validity of the test and makes it inappropriate as a specific diagnostic tool for identifying the SARS-CoV-2 virus.
- Most likely, Corman-Drosten’s work has not been reviewed by experts, which makes the test inadequate as a specific diagnostic tool for the identification of SARS-CoV-2 virus.
- We find severe conflicts of interest for at least four authors, in addition to the fact that two of the authors of Corman-Drosten (Christian Drosten and Chantal Reusken) are members of the editorial board of Eurosurveillance.
A conflict of interest was added on July 29, 2020 (Olfert Landt is CEO of TIB-Molbiol; Marco Kaiser is a senior researcher at GenExpress and a scientific advisor for TIB-Molbiol); this conflict of interest was not stated in the original version (and is still missing in the PubMed version); TIB-Molbiol is the company that was the “first” to produce PCR (Light Mix) kits based on the protocol published in the Corman-Drosten manuscript and, in their own words, distributed these PCR test kits before the paper was even submitted to publication; in addition, Victor Corman and Christian Drosten did not mention their second affiliation: the “Labor Berlin” commercial testing laboratory.
In light of the re-examination of the test protocol for the identification of SARS-CoV-2 described in the paper Corman-Drosten, we have identified errors and erroneous conclusions, which make the SARS-CoV-2 PCR test useless.
Authors Corman Drosten et al. they introduced the background for their work as follows:
“The epidemic of the new coronavirus (2019-nCoV) is a challenge for public health laboratories because isolated virus samples are not available, while there is growing evidence that the outbreak is more widespread than previously thought, and international travel is already taking place. “
No virus was isolated
The “new SARS-CoV-2 coronavirus” in the paper is composed of theoretical sequences (in silico), provided by a laboratory in China, because at that time the authors did not have any infectious SARS-CoV-2 control material (“live “) or inactivated, nor the isolated genomic RNA of the virus. In fact, to date, no author validation has been performed based on any isolated SARS-CoV-2 virus or its complete RNA.
According to the paper:
“Our goal was to develop and implement a robust diagnostic methodology for use in public health laboratories without viral material available.”
Here two things must be emphasized: a) the development and b) the implementation of a diagnostic test for laboratory use.
These goals cannot be achieved without real viral material available. However, silico (theoretical) sequences were used to develop the RT-PCR testing methodology, with a model based on the assumption that the new virus would be very similar to the 2003 SARS-CoV.
The entire model designed by Corman-Drosten contains severe errors, as the test cannot differentiate between the whole virus and viral fragments.
The test cannot be used as a diagnosis for SARS viruses.
Sources:
www.corodok.de/drosten-dissertation-verschluss/
www.rubikon.news/artikel/der-goldjunge
cormandrostenreview.com/report/
www.corodok.de/vergiftete-geschenke-dritte/
www.corodok.de/drosten-dissertation-kuehbacher/
www.corodok.de/kuehbacher-goethe-uni/

