Moderna (MRNA) said on Sunday that it has been awarded up to $472 million by the U.S. Biomedical Advanced Research and Development Authority (BARDA) to support late stage clinical development of its mRNA vaccine candidate (mRNA-1273) against COVID-19.
The U.S. grant will be used to fund the drugmaker’s expanded Phase 3 study of mRNA-1273, which includes 30,000 participants in the U.S and is scheduled to start on July 27. After having already received $483 million from BARDA earlier this year to support early clinical development of the investigational vaccine, the total value of the award is now about $955 million.
“We thank BARDA for this continued commitment to mRNA-1273, our vaccine candidate against COVID-19.” said Moderna’s CEO Stéphane Bancel. “Encouraged by the Phase 1 data, we believe that our mRNA vaccine may aid in addressing the COVID-19 pandemic and preventing future outbreaks.”
The primary endpoint of the randomized, placebo-controlled Phase 3 trial will be the prevention of symptomatic COVID-19 disease. Key secondary endpoints include the prevention of severe COVID-19 disease as defined by the need for hospitalization and prevention of infection by SARS-CoV-2.
Moderna reiterated that the company remains on track to deliver about 500 million doses per year, and up to 1 billion doses per year, beginning in 2021. Initial funding of $1.3 billion for Moderna to begin producing mRNA-1273 was secured from investors in the company’s most recent public equity offering in May 2020.
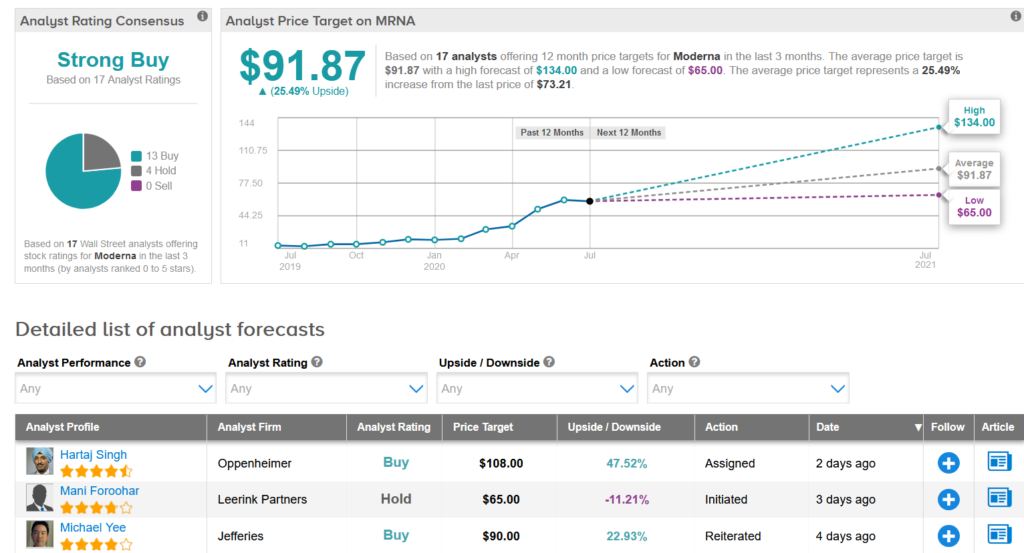
Shares in Moderna have surged 276% so far this year, and Wall Street analysts have a Strong Buy consensus on the stock’s outlook. The $91.87 average price target suggests an additional 25% upside potential lies ahead. (See MRNA stock analysis on TipRanks).
In a bullish note, five-star analyst Hartaj Singh at Oppenheimer on Friday assigned a Buy rating on the stock with a $108 price target suggesting shares have room to gain another 48% over the coming year.
“We do see a tricky period ahead for MRNA as logistics and discussions of commercialization (e.g., pricing) intensify,” Singh wrote in a note to investors on July 24. “We continue to see Moderna as well-positioned to navigate these waters, based on the company’s impressive execution in the clinic, thoughtful commentary on the clinical/commercial landscape, and delivered results. Hence, we would buy MRNA.”
Related News:
Pfizer, BioNTech Ink UK Supply Deal For 30M Covid-19 Vaccine Doses
Moderna Soars 16% As Covid-19 Vaccine Shows Strong Immune Response
GSK Buys 10% Stake In Germany’s CureVac To Develop mRNA Vaccines

